Question
In: Chemistry
The center of a cubic hole is found ________ at the center of a simple cubic...
The center of a cubic hole is found ________
| at the center of a simple cubic lattice. |
| at eight sites within a face-centered cubic lattice. |
| on the edges of a simple cubic lattice. |
| at the center of a face-centered cubic lattice. |
| on the faces of a body-centered cubic lattice. |
Solutions
Expert Solution
| Name | Primitive cubic | Body-centered cubic | Face-centered cubic |
|---|---|---|---|
| Pearson symbol | cP | cI |
cF |
| Unit cell | 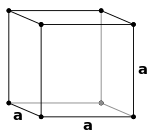 |
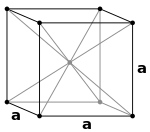 |
|
A simple cubic unit cell has a single cubic void in the center.
A body-centered cubic unit cell has six octahedral voids located at the center of each face of the unit cell, for a total of three net octahedral voids. Additionally, there are 36 tetrahedral voids located in an octahedral spacing around each octahedral void, for a total of eighteen net tetrahedral voids. These tetrahedral voids are not local maxima and are not technically voids, but they do occasionally appear in multi-atom unit cells.
A face-centered cubic unit cell has eight tetrahedral voids located slightly towards the center from each corner of the unit cell, for a total of eight net tetrahedral voids. Additionally, there are twelve octahedral voids located at the center of edge of the unit cell as well as one octahedral hole in the very center, for a total of four net octahedral voids.
Answer-> at the center of a face-centered cubic lattice
Related Solutions
The singularity at the center of a black hole is predicted to be a region of...
What is the mass of the black hole at the center of our galaxy if a...
Which of these are actual evidence for the existence of a black hole in the center...
1. What is the evidence for a black hole at the center of the Milky Way?...
Describe the evidence for a supermassive black hole at the center of our Galaxy.
What is the Schwarzschild radius of the black hole at the center of the Milky Way?...
The supermassive black hole in the center of the Milky Way has a mass of roughly...
A sphere with a hole drilled through its center is threaded onto a wire. The wire...
A The U.S.S. Enterprise (NCC‑1701) approaches an unknown system with a Black Hole at the center....
How do astronomers estimate the mass of the supermassive black hole at the center of our...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...

 queen_honey_blossom answered 3 months ago
queen_honey_blossom answered 3 months ago