Question
In: Chemistry
Copper reacts with dilute nitric acid according to: 3 Cu(s) + 8 HNO3(aq) ? 3 Cu(NO3)2(aq)...
Copper reacts with dilute nitric acid according to:
3 Cu(s) + 8 HNO3(aq) ? 3 Cu(NO3)2(aq) + 2 NO(g) + 4 H2O(l)
If a copper penny weighing 3.045-g is dissolved in 37.23 mL of 3.750 M nitric acid and the resultant solution is diluted to 50.00 mL in a volumetric flask, what is the final concentration of NO3- in the solution?
Solutions
Expert Solution
This problem can be easily solved by going through the following steps
The given reaction is
3 Cu(s) + 8 HNO3(aq) 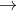 3
Cu(NO3)2(aq) + 2 NO(g) + 4 H2O(l)
3
Cu(NO3)2(aq) + 2 NO(g) + 4 H2O(l)
Step -1: Finding the moles of Cu and HNO3
Moles of Cu = 3.045 g/63.55gmol-1 = 0.04791 mol
Moles of HNO3 = MxV = 3.750molL-1x37.23 x 10-3 L = 0.1396 mol
Step -2: Finding the limiting reagent
3 moles of Cu reacts with 8 moles of HNO3 in the balanced chemical reaction.
moles of HNO3 /moles of Cu = 0.1396 /0.04791 = 2.914 which is greater than the molar coefficient ratio 8/3.
Hence Cu is the limiting regent and is exhausted first leaving the excess of HNO3 in the solution.
Hence the final concentration of NO3- will be due to both HNO3 and Cu(NO3)2, beause both are completely ionised in the solution.
Step-3: Finding the moles of Cu(NO3)2 formed and excess moles of HNO3
Moles of Cu(NO3)2 formed = (3/3)x0.0479 mol = 0.0479 mol
Moles of HNO3 reacted with Cu = (8/3)x0.0479 = 0.1277 mol
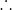 moles of
HNO3 remain unreacted in the solution = 0.1396 mol - 0.1277 mol =
0.0119mol
moles of
HNO3 remain unreacted in the solution = 0.1396 mol - 0.1277 mol =
0.0119mol
Step -3: Calculating the moles of NO3- in the final solution
moles of NO3- in 0.0479 mol of Cu(NO3)2 = 2x0.0479 mol = 0.0958 mol
moles of NO3- in 0.0119mol of unreacted HNO3 = 1x0.0119mol = 0.0119 mol
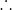 Total moles
of NO3- in the final solution = 0.0958
mol+0.0119 mol = 0.1077 mol
Total moles
of NO3- in the final solution = 0.0958
mol+0.0119 mol = 0.1077 mol
Step-4: Calculating the final concentration of NO3-
Total volume of the solutin = 50.00mL = 50.00x10-3 L
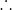 final
concentration of NO3- = 0.1077 mol/50.00x10-3
L= 2.154M (answer)
final
concentration of NO3- = 0.1077 mol/50.00x10-3
L= 2.154M (answer)
Related Solutions
Homework Text bOOK Here is the reaction Cu (s) + 4 HNO3 (aq) >>> Cu(NO3)2 (aq)...
identify the oxidizing agent in the reaction: Fe(s)+Cu(No3)2(aq)->Fe(NO3)2(aq)+Cu(s)
the equation: 2agn03 (aq) +cu (s) heat 2 ag (s) + Cu (NO3) 2 (aq) is...
Preparation of Copper(I) Chloride Reactions: 1) Cu(S) + 4HNO3(aq) -> Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l) 2)...
Given the reaction: 2 Al (s) + 6 HNO3 (aq) ® 2 Al(NO3)3 (aq) + 3...
Cu(s)+HNo3(aq)- Cu(NO3)2(aq)+NO2(g)+H2O 1. which chemical species is the limiting reaggent? 2.which chemical spcies is the non-limiting...
Consider the balanced chemical reaction Cu(s) + 2AgNO3(aq) a 2Ag(s) + Cu(NO3)2(aq) a) Write the Complete...
Balance the following chemical equation: Cu(s)+HNO3(aq)=Cu(NO3)2+NO(g)+H2O(l). I missed this question on a quiz after trying to...
Consider the reaction: 2 Na3PO4(aq) + 3 Cu(NO3)2(aq) → Cu3(PO4)2(s) + 6 NaNO3(aq) What mass of...
Preparation of CuCl Reactions: 1) Cu(S) + 4HNO3(aq) -> Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l) 2) 2HNO3(aq)...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 4 months ago
queen_honey_blossom answered 4 months ago