Question
In: Chemistry
How many unique 1H NMR signals exist in the spectrum of the following compound?
How many unique 1H NMR signals exist in the spectrum of the following compound?
Solutions
Expert Solution
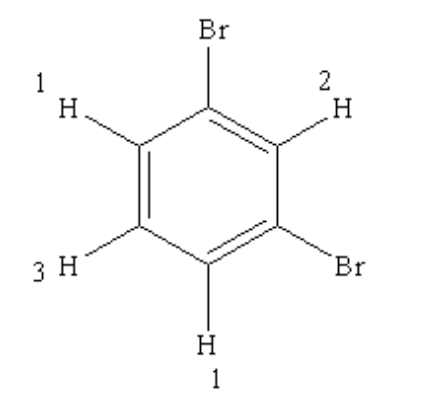
Three types of \({ }^{1} \mathrm{H}\) -NMR signal s exist in the spectrum of this compound.
There are a total of 3 types of protons are present in this compound, so 3 types of signal s are found.
Related Solutions
How many unique 1 H NMR signals exist in the spectrum of the following compound: 1,3-dibromobenzene?
How many unique 1 H NMR signals exist in the spectrum of the
following compound: 1,3-dibromobenzene?
1H NMR spectrum A corresponds to a molecule with the formula C3H5Cl.
1H NMR spectrum A corresponds to a molecule with the formula C3H5Cl. The compound shows significant IR bands at 730 (see Problem 53 of Chapter 11), 930, 980, 1630, and 3090 cm-1.
(a) Deduce the structure of the molecule.
(b) Assign each NMR signal to a hydrogen or group of hydrogens.
(c) The “doublet” at δ = 4.05 ppm has J ≈ 6 Hz. Is this in accord with your assignment in (b)?
(d) This “doublet,” upon five-fold expansion, becomes...
The 1H NMR of ethanol in CDCl3 will consist of three signals in the spectra: a...
The 1H NMR of ethanol in CDCl3 will consist of three signals in
the spectra: a triplet at 1.25 ppm, a quartet at 3.72 ppm, and a
broad singlet at 1.32 ppm. In DMSO-d6(deuterated DMSO) the broad
singlet is found at 4.63 ppm. Why is there such a dramatic change
in the singlet’s position (clearly describe what is happening with
structure)?
How would the 1H NMR spectrum of butane and butanone differ from each other? Sketch the...
How would the 1H NMR spectrum of butane and butanone
differ from each other? Sketch the coupled 1H spectrum
of each and explain the splitting patterns you would expect.
How would you use a DEPT spectrum and a {1H}13C spectrum to deduce how many quaternary...
How would you use a DEPT spectrum and a
{1H}13C spectrum to deduce how many
quaternary carbon atoms you have?
Identify the compound A (C5H10O) with the proton NMR spectrum shown. Compound A has IR absorptions...
Identify the compound A (C5H10O) with the proton NMR spectrum
shown. Compound A has IR absorptions at 3200–3600 cm–1 (strong,
broad), 1676 cm–1 (weak), and 965 cm–1, and also has 13C NMR
absorptions (attached protons in parentheses) at δ 17.5 (3), δ 23.3
(3), δ 68.8 (1), δ 125.5 (1), and δ 135.5 (1). Compound A may be
resolved into enantiomers; draw one molecule of A, omitting
wedge/dash bonds.
A compound has a molecular formula of C4H6O2 and exhibits the following 13C NMR spectrum. δ...
A compound has a molecular formula of
C4H6O2 and exhibits the following
13C NMR spectrum. δ 51.32, 44.17
Which of the compounds listed below would be consistent with
this structure?
A compound has a molecular formula of
C5H8O2 and exhibits the following
13C NMR spectrum.
δ 199.83, 197.54, 29.22, 23.70, 6.95
Which of the compounds listed below would be consistent with
this structure?
A compound has a molecular formula of
C4H6O2 and exhibits the following
13C NMR spectrum.
δ 51.32,...
The proton NMR spectrum for a compound with the formula C7H14O is shown below along with...
The proton NMR spectrum for a compound with the formula C7H14O
is shown below along with carbon-13 spectral data in tabular form.
(It may be necessary to expand (zoom) some of the 1H signals to
view spin-spin splitting details.)
This compound exhibits strong infrared absorption at
1717 cm-1, but the 1500-1650 cm-1 region is
empty.
13C Data
Normal Carbon
DEPT-135
DEPT-90
Proton Shift
Relative Area
13.8
Positive
No peak
0.91
3.2
17.2
Negative
No peak
1.09
5.8
18.2
Positive
No...
What compound is used as a standard for location of NMR signals? Select one: a. acetone,...
What compound is used as a standard for location of NMR
signals?
Select one:
a. acetone, CH3COCH3
b. silicon grease
c. water
d. deuterated water, D2O
e. deuterated chloroform, CDCl3
f. trimethylsilane, (CH3)4Si
g. chloroform, CHCl3
In NMR, what kind of information do you get when you collect a 15N-1H HSQC spectrum...
In NMR, what kind of information do you get when you collect a
15N-1H HSQC spectrum of a protein?
a. Interactions between protons < 5Å apart.
b.A bunch of peaks
c.The 2D spectrum of all the amide N's and their attached
protons.
d. A 3D spectrum
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
ADVERTISEMENT
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago