Question
In: Chemistry
please explain and label the signals of ethylbenzene c6h14o proton nmr and IR. thank you..
please explain and label the signals of ethylbenzene c6h14o proton nmr and IR. thank you..
Solutions
Expert Solution
Hi, I cannot see any spectrum uploaded from you in this question. To explain this I am using a simulated spectrum for NMR. I have generated this spectrum from ChemSketch. Here it is:
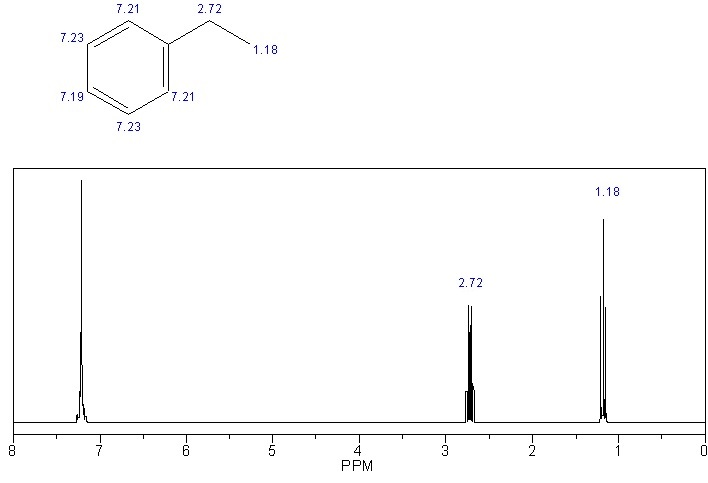
The CH3 group at the terminal gives a triplet since it is next to a CH2 group. The triplet appears at 1.18 ppm.
Since the CH2 group is next to a CH3 it gives a quartet and the quartet appears at 2.72. This signal is downfield since the CH2 group is next to the aromatic ring.
The aromatic ring protons all appear as a multiplet at 7.19-7.23. Aromatic protons usually appear at ppm values around 6.5 to 7.5. Now come to the IR.
I took the IR spectrum from the NIST website which contains the data for many chemicals.
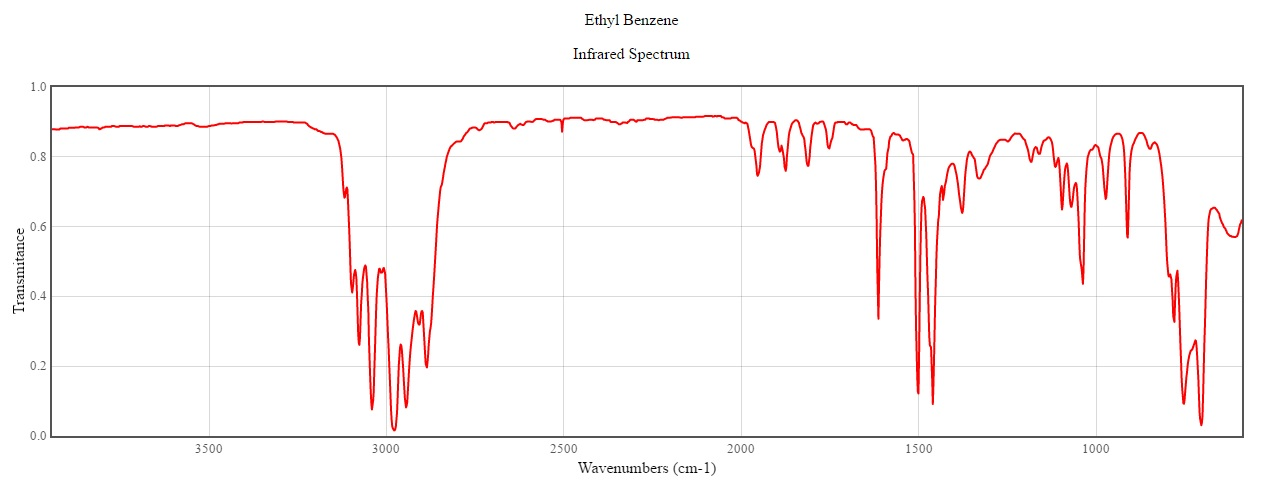
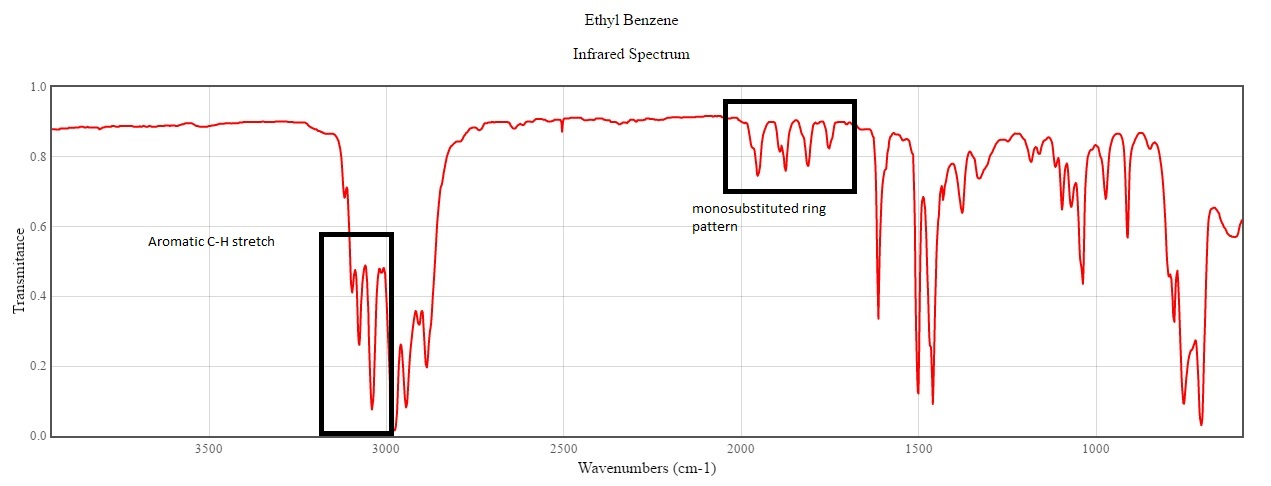
Since the molecule does not have any functional group, the IR spectrum is not of much help. All that we can interpret is the following:
A monosubstituted ring pattern (1667-2000) which is very decisive and the aromatic C-H stretch which occurs above 3000.
Related Solutions
Find the IR and NMR spectra of Ibuprofen from the internet. Label the peaks and please...
What proton nmr signals (at high field giving little overlap of signals) would be given by...
Identify the compound A (C5H10O) with the proton NMR spectrum shown. Compound A has IR absorptions...
Explain the proper techniques for cleaning the NMR tubes. Explain NMR and IR spectroscopy and what...
State the theory and explain how NMR and IR spectroscopy work.
1. Explain how each spectroscopy (IR, Mass spec, and NMR) method works- what are you observing?...
What are the three things the NMR spectra tell you for each proton?
List the signals you would expect to in an IR as evidence that there is 2,3-dibromo-3-...
How is NMR techniques better than IR? please provide detailed pros and cons. Many thanks
Explain what the different peaks mean and compare to what a proton nmr of the same substance would be?
- A faulty model rocket moves in the xy-plane (the positive y-direction is vertically upward). The rocket's...
- A charged nonconducting rod, with a length of 3.68 m and a cross-sectional area of 2.79...
- Please list your 3 most favorite brands and say why. 1. 2. 3. Please list your...
- Six years from today you need $10,000. You plan to deposit $1,600 annually, with the first...
- 1. How did IBM become the dominant IT industry leader? 2. What changed that knocked IBM...
- Rental Shop A. Create an abstract class named SummerSportRental that is to be used with for...
- 1-Suppose a Styrofoam cup that weighs 5 grams was used for this experiment in place of...
 queen_honey_blossom answered 10 hours ago
queen_honey_blossom answered 10 hours ago