Question
In: Chemistry
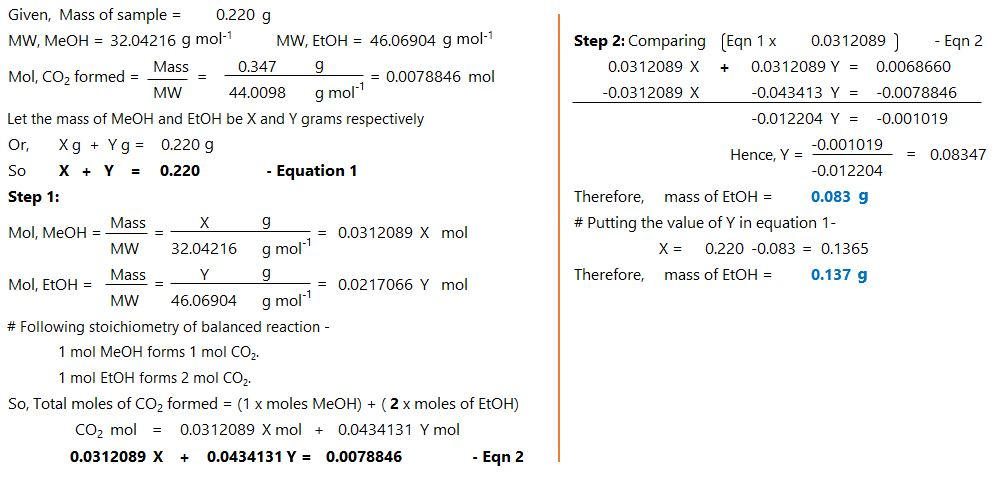
An organic liquid is a mixture of methyl alcohol (CH3OH) and ethyl alcohol (C2H5OH). A 0.220-g...
An organic liquid is a mixture of methyl alcohol (CH3OH) and
ethyl alcohol (C2H5OH). A 0.220-g sample of the liquid is burned in
an excess of O2(g) and yields 0.347 g CO2(g) (carbon
dioxide).
Set up two algebraic equations, one expressing the mass of carbon
dioxide produced in terms of each reagent and the other expressing
the mass of sample burned in terms of each reagent.
What is the mass of methyl alcohol (CH3OH) in the sample?
Solutions
Related Solutions
An organic liquid is a mixture of methyl alcohol (CH3OH) and ethyl alcohol (C2H5OH). A 0.220-g...
An organic liquid is a mixture of methyl alcohol (CH3OH) and
ethyl alcohol (C2H5OH). A 0.220-g sample of the liquid is burned in
an excess of O2(g) and yields 0.380 g CO2(g) (carbon dioxide).
Set up two algebraic equations, one expressing the mass of
carbon dioxide produced in terms of each reagent and the other
expressing the mass of sample burned in terms of each reagent.
What is the mass of methyl alcohol (CH3OH) in the sample?
An organic liquid is a mixture of methyl alcohol (CH3OH) and ethyl alcohol (C2H5OH). A 0.220-g...
An organic liquid is a mixture of methyl alcohol (CH3OH) and
ethyl alcohol (C2H5OH). A 0.220-g sample of the liquid is burned in
an excess of O2(g) and yields 0.376 g CO2(g) (carbon dioxide).
Set up two algebraic equations, one expressing the mass of
carbon dioxide produced in terms of each reagent and the other
expressing the mass of sample burned in terms of each reagent. What
is the mass of methyl alcohol (CH3OH) in the sample?
An organic liquid is a mixture of methyl alcohol (CH3OH) and ethyl alcohol (C2H5OH). A 0.220-gsample...
An organic liquid is a mixture of methyl alcohol (CH3OH) and
ethyl alcohol (C2H5OH). A 0.220-gsample of the liquid is burned in
an excess of O2(g) and yields 0.348 g CO2(g) (carbon dioxide).
Set up two algebraic equations, one expressing the mass of
carbon dioxide produced in terms of each reagent and the other
expressing the mass of sample burned in terms of each reagent.
What is the mass of methyl alcohol (CH3OH) in the sample?
A 384.0 g/hr stream of liquid methyl alcohol, also called methanol, (CH3OH) at 7.60 atm and...
A 384.0 g/hr stream of liquid methyl alcohol, also called
methanol, (CH3OH) at 7.60 atm and 10.0°C was held at
constant pressure, vaporized and brought to 564.0°C. At what rate
must heat be supplied to this system? Assume that methyl alcohol
vapor behaves ideally for the temperature range and pressure
given.
A fuel mixture consists of 90% octane (C8H18) and 10% ethyl alcohol (C2H5OH), by volume. This...
A fuel mixture consists of 90% octane
(C8H18) and 10% ethyl alcohol
(C2H5OH), by volume. This fuel mixture is
burned with 200% theoretical dry air in a combustion chamber at
atmospheric pressure.
a) Write the balanced reaction equation for complete combustion
of this fuel mixture.
b) Determine the theoretical air-fuel ratio.
c) Actual air-flow rate for a fuel mixture flow rate of 5 kg/s.
d) Calculate the molar mass of the mixture product gases.
A fuel mixture consists of 90% octane (C8H18) and 10% ethyl alcohol (C2H5OH), by volume. This...
A fuel mixture consists of 90% octane
(C8H18) and 10% ethyl alcohol
(C2H5OH), by volume. This fuel mixture is
burned with 200% theoretical dry air in a combustion chamber at
atmospheric pressure.
a) Write the balanced reaction equation for complete combustion
of this fuel mixture.
b) Determine the theoretical air-fuel ratio.
c) Actual air-flow rate for a fuel mixture flow rate of 5
kg/s.
d) Calculate the molar mass of the mixture product gases.
A 5 g mixture of ethanol (C2H5OH) and methanol (CH3OH) reacts with excess oxygen. If this...
A 5 g mixture of ethanol (C2H5OH) and
methanol (CH3OH) reacts with excess oxygen. If this
combustion releases 125.46 kJ of heat, what mass of ethanol is in
the mixture? Assume that mixing does not affect any enthalpy
values.
C2H5OH(l) + 3 O2(g) --> 2
CO2(g) + 3 H2O(g)
-277.63
-393.5 -241.83 (kJ/mol)
CH3OH(l) + 1.5 O2(g) -->
CO2(g) + 2 H2O(g)
-201.2
-393.5 -241.83 (kJ/mol)
The formation of ethyl alcohol (C2H5OH MM = 46.08 g/mol) by the fermentation of glucose (C6H12O6...
The formation of ethyl alcohol (C2H5OH MM
= 46.08 g/mol) by the fermentation of glucose
(C6H12O6 MM = 180.18 g/mol) may be
represented by:
C6H12O6 →
2C2H5OH + 2CO2
If a particular glucose fermentation process is 87.0% efficient,
how many grams of glucose would be required for the production of
56.0 g of ethyl alcohol (C2H5OH)?
An experiment requires 40.3 of ethyl alcohol. If the density of ethyl alcohol is 0.790 g/(cm3),...
An experiment requires 40.3 of ethyl alcohol. If the density of
ethyl alcohol is 0.790 g/(cm3), what is the mass of 40.3
mL of ethyl alcohol
A) An aqueous solution of methyl alcohol is made by transfering 2.39 mL of liquid methyl...
A) An aqueous solution of methyl alcohol is made by transfering
2.39 mL of liquid methyl alcohol to a 100. mL volumetric flask, and
then adding enough water to fill the flask to the mark. What is the
volume/volume percentage of methyl alcohol in the solution?
B) An aqueous solution of acetone is made by
transfering 7.17 mL of liquid
acetone to a 100. mL volumetric
flask, and then adding enough water to fill the flask to the mark.
What...
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
ADVERTISEMENT
 queen_honey_blossom answered 1 month ago
queen_honey_blossom answered 1 month ago