Question
In: Chemistry
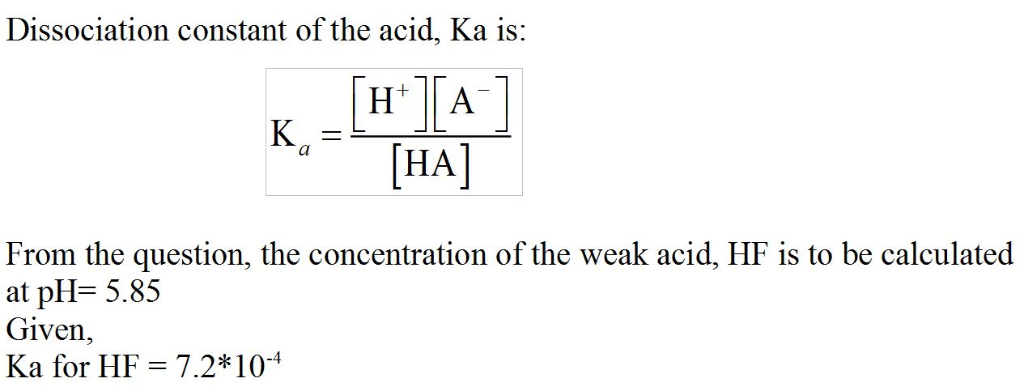
Hyrdrofluoric acid (Ka = 7.2 x 10-4), is used in a wide range of commercial processes....
Hyrdrofluoric acid (Ka = 7.2 x 10-4), is used in a
wide range of commercial processes. HF is used for glass etching
and the synthesis of pharmaceutical drugs and compounds such as
teflon. One issue with HF is its corrosive nature, "Aqueous
hydrofluoric acid is a contact-poison with the potential for deep,
initially painless burns and ensuing tissue death." (from Wikepedia
page on hydrofluoric acid).
The flouride ion is often used in dental procedures, as the F- can
react with tooth enamel to form highly stable mineral
fluorohydroxyapatite (which is less likely to break down in the
mouth than "normal" enamel). One common form of F-
delivery is fluoride varnish, which has a F-
concentration of around 1.778 M.
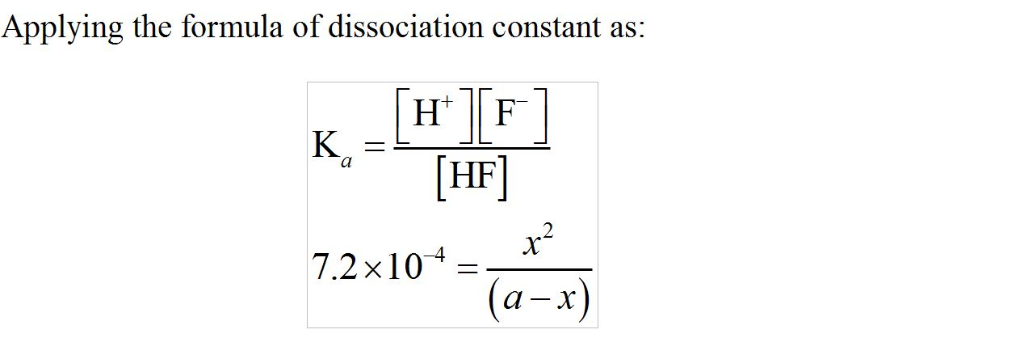
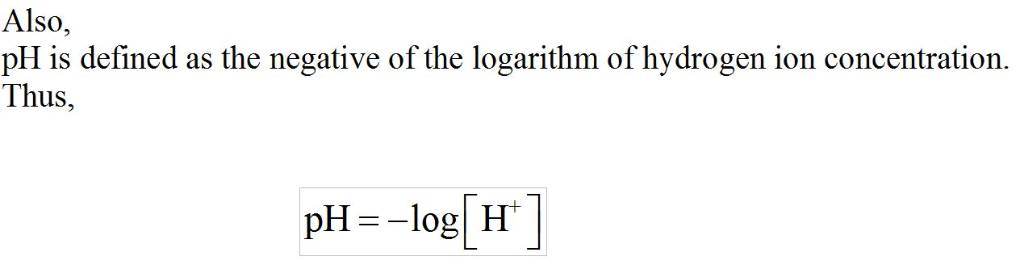
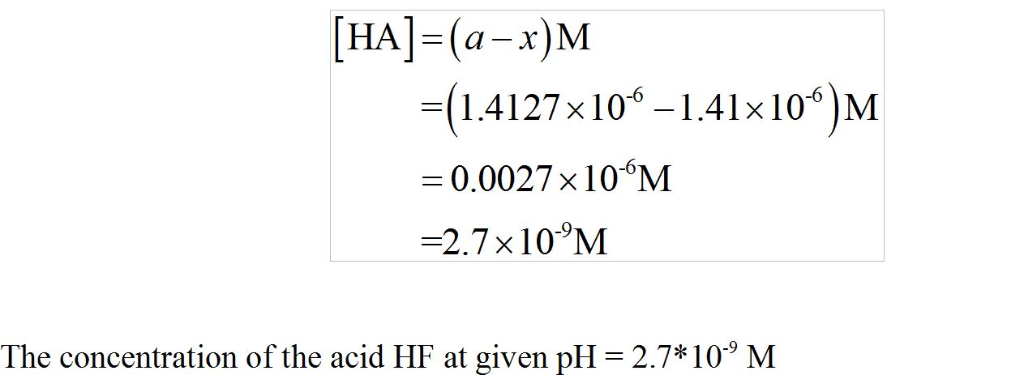
Given what you know of the acid base chemistry of HF, what is the
concentration of HF in an aqueous solution with a pH of 5.85?
Solutions
Related Solutions
An aqueous solution contains a mixture of .175 M HF (Ka = 7.2 x 10-4) and...
formic acid is a weak acid Ka= 1.9 x 10^-4 . Calculate the ph of a...
Lactic acid (HC3H5O3), a weak monoprotic acid with a Ka = 1.4 x 10-4, is titrated...
For nitrous acid, HNO2, the value of Ka is 4.0 x 10-4. a. What is the...
The Ka for Formic acid (HCO2H) is 1.8 x 10^-4. What is the pH of a...
1. Ka for hydrocyanic acid, HCN, is 4.00×10-10. Ka for acetylsalicylic acid (aspirin), HC9H7O4, is 3.00×10-4. Ka...
1)Lactic acid (HC3H5O3), a weak monoprotic acid with a Ka = 1.4 x 10-4, is titrated...
Arsenic acid (H3AsO4) has Ka values of 2.5 x 10–4, 5.6 x 10–8, and 3 x...
The weak acid HZ has a Ka of 2.55 x 10-4. Calculate the pH of a...
Calculate [H3O + ] for a 2.5 x 10-4 M solution of weak acid (Ka =...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...

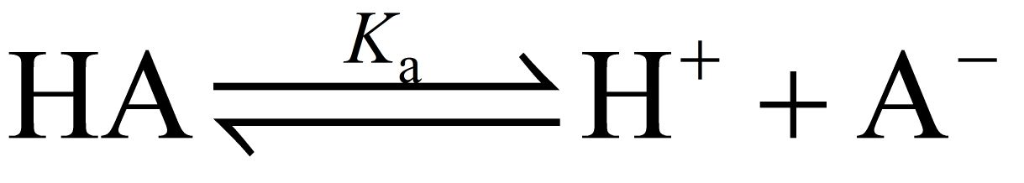
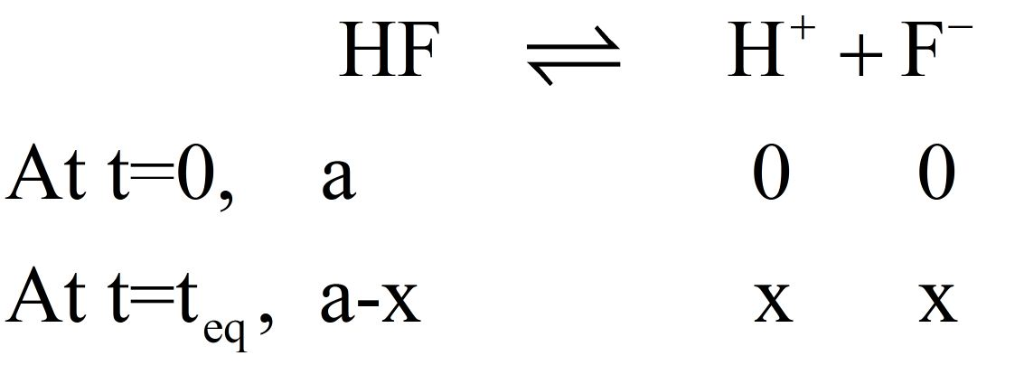
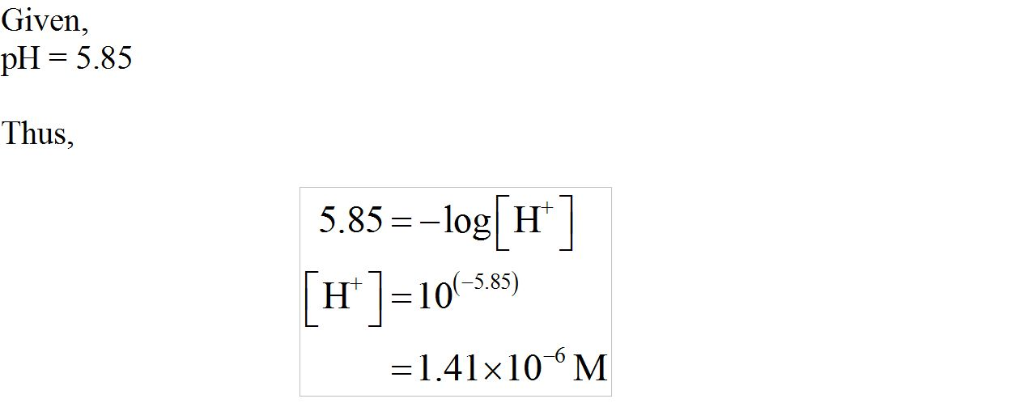

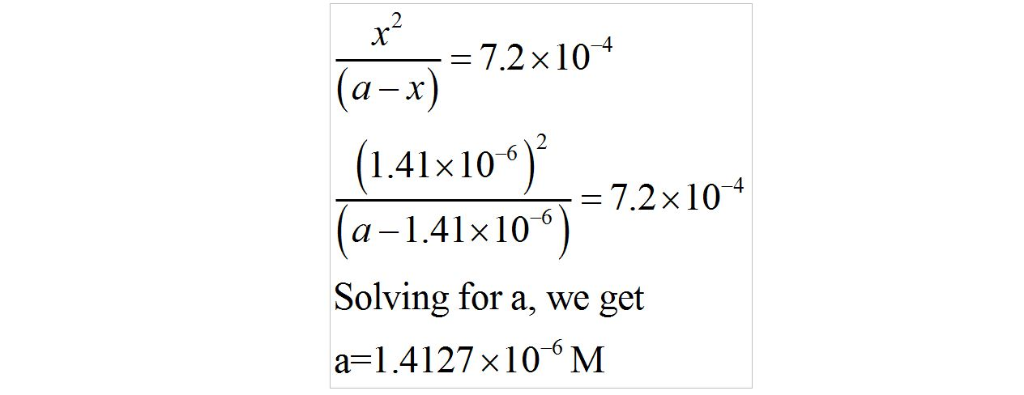

 queen_honey_blossom answered 2 months ago
queen_honey_blossom answered 2 months ago