Question
In: Chemistry
During an annealing heat treatment, the final size reached by the grains is controlled by both...
During an annealing heat treatment, the final size reached by the grains is controlled by both temperature and the time at temperature.
Describe how time and temperature control grain size in annealing (for example what leads to a larger grain size)?
What is the general formula/relationship that describes the temperature-dependence seen? Explain the mechanism controlling this T-dependence.
Solutions
Expert Solution
Heat treating is a group of industrial and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve a desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening,tempering, normalizing and quenching. It is noteworthy that while the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.

Heat treating furnace at 1,800 °F (980 °C)
Physical processes
Metallic materials consist of a microstructure of small crystals called "grains" or crystallites. The nature of the grains (i.e. grain size and composition) is one of the most effective factors that can determine the overall mechanical behavior of the metal. Heat treatment provides an efficient way to manipulate the properties of the metal by controlling the rate of diffusion and the rate of cooling within the microstructure. Heat treating is often used to alter the mechanical properties of a metallic alloy, manipulating properties such as thehardness, strength, toughness, ductility, and elasticity.
There are two mechanisms that may change an alloy's properties during heat treatment: the formation of martensite causes the crystals to deform intrinsically, and the diffusion mechanism causes changes in the homogeneity of the alloy.
The crystal structure consists of atoms that are grouped in a very specific arrangement, called a lattice. In most elements, this order will rearrange itself, depending on conditions like temperature and pressure. This rearrangement, called allotropy or polymorphism, may occur several times, at many different temperatures for a particular metal. In alloys, this rearrangement may cause an element that will not normally dissolve into the base metal to suddenly become soluble, while a reversal of the allotropy will make the elements either partially or completely insoluble.
When in the soluble state, the process of diffusion causes the atoms of the dissolved element to spread out, attempting to form a homogenous distribution within the crystals of the base metal. If the alloy is cooled to an insoluble state, the atoms of the dissolved constituents (solutes) may migrate out of the solution. This type of diffusion, called precipitation, leads to nucleation, where the migrating atoms group together at the grain-boundaries. This forms a microstructure generally consisting of two or more distinct phases. Steel that has been cooled slowly, for instance, forms a laminated structure composed of alternating layers of ferrite and cementite, becoming soft pearlite.
Unlike iron-based alloys, most heat treatable alloys do not experience a ferrite transformation. In these alloys, the nucleation at the grain-boundaries often reinforces the structure of the crystal matrix. These metals harden by precipitation. Typically a slow process, depending on temperature, this is often referred to as "age hardening".
Many metals and non-metals exhibit a martensite transformation when cooled quickly(with external media like oil,polymer,water etc.). When a metal is cooled very quickly, the insoluble atoms may not be able to migrate out of the solution in time. This is called a "diffusionless transformation." When the crystal matrix changes to its low temperature arrangement, the atoms of the solute become trapped within the lattice. The trapped atoms prevent the crystal matrix from completely changing into its low temperature allotrope, creating shearing stresses within the lattice. When some alloys are cooled quickly, such as steel, the martensite transformation hardens the metal, while in others, like aluminum, the alloy becomes softer.
 Allotropes of iron, showing the
differences in lattice structures between alpha iron (low
temperature) and gamma iron (high temperature). The alpha iron has
no spaces for carbon atoms to reside, while the gamma iron is open
to free movement of small carbon atoms.
Allotropes of iron, showing the
differences in lattice structures between alpha iron (low
temperature) and gamma iron (high temperature). The alpha iron has
no spaces for carbon atoms to reside, while the gamma iron is open
to free movement of small carbon atoms.
Effects of time and temperature
Proper heat treating requires precise control over temperature, time held at a certain temperature and cooling rate.
With the exception of stress-relieving, tempering, and aging, most heat treatments begin by heating an alloy beyond the upper transformation (A3) temperature. This temperature is referred to as an "arrest" because at the A3 temperature nothing happens. Therefore, the alloy must be heated above the temperature for a transformation to occur. The alloy will usually be held at this temperature long enough for the heat to completely penetrate the alloy, thereby bringing it into a complete solid solution.
Because a smaller grain size usually enhances mechanical properties, such as toughness, shear strength and tensile strength, these metals are often heated to a temperature that is just above the upper critical temperature, in order to prevent the grains of solution from growing too large. For instance, when steel is heated above the upper critical temperature, small grains of austenite form. These grow larger as temperature is increased. When cooled very quickly, during a martensite transformation, the austenite grain-size directly affects the martensitic grain-size. Larger grains have large grain-boundaries, which serve as weak spots in the structure. The grain size is usually controlled to reduce the probability of breakage.
The diffusion transformation is very time-dependent. Cooling a metal will usually suppress the precipitation to a much lower temperature. Austenite, for example, usually only exists above the upper critical temperature. However, if the austenite is cooled quickly enough, the transformation may be suppressed for hundreds of degrees below the lower critical temperature. Such austenite is highly unstable and, if given enough time, will precipitate into various microstructures of ferrite and cementite. The cooling rate can be used to control the rate of grain growth or can even be used to produce partially martensitic microstructures. However, the martensite transformation is time-independent. If the alloy is cooled to the martensite transformation (Ms) temperature before other microstructures can fully form, the transformation will usually occur at just under the speed of sound.
When austenite is cooled slow enough that a martensite transformation does not occur, the austenite grain size will have an effect on the rate of nucleation, but it is generally temperature and the rate of cooling that controls the grain size and microstructure. When austenite is cooled extremely slow, it will form large ferrite crystals filled with spherical inclusions of cementite. This microstructure is referred to as "sphereoidite." If cooled a little faster, then coarse pearlite will form. Even faster, and fine pearlite will form. If cooled even faster, bainite will form. Similarly, these microstructures will also form if cooled to a specific temperature and then held there for a certain time.[15]
Most non-ferrous alloys are also heated in order to form a solution. Most often, these are then cooled very quickly to produce a martensite transformation, putting the solution into a supersaturated state. The alloy, being in a much softer state, may then be cold worked. This cold working increases the strength and hardness of the alloy, and the defects caused by plastic deformation tend to speed up precipitation, increasing the hardness beyond what is normal for the alloy. Even if not cold worked, the solutes in these alloys will usually precipitate, although the process may take much longer. Sometimes these metals are then heated to a temperature that is below the lower critical (A1) temperature, preventing recrystallization, in order to speed-up the precipitation.
 Time-temperature transformation
(TTT) diagram for steel. The red curves represent different cooling
rates (velocity) when cooled from the upper critical (A3)
temperature. V1 produces martensite. V2 has pearlite mixed with
martensite, V3 produces bainite, along with pearlite and
matensite.
Time-temperature transformation
(TTT) diagram for steel. The red curves represent different cooling
rates (velocity) when cooled from the upper critical (A3)
temperature. V1 produces martensite. V2 has pearlite mixed with
martensite, V3 produces bainite, along with pearlite and
matensite.
Many metal fabrication processes involve cold-working, such as forming sheet and plate, wire drawing, and deep drawing. As the amount of cold-working increases, the ductility of the metal decreases. At some point it is necessary to anneal the metal if continued cold-working is required. The purpose of the anneal process is to increase the metal’s ductility.
During cold-working there is damage to the metal on the microscopic level that causes a metal’s yield strength to increase and its ductility to decrease. After a certain amount of cold work, a metal cannot be cold worked anymore without cracking. The specific amount of cold work that a particular metal can withstand depends on its composition.
Annealing a metal restores it to its pre-cold-worked state, whereupon, the metal can be subjected to further cold working. During the annealing process, metallurgical changes occur within the metal that results in a reduction of the metal’s yield strength and an increase in its ductility. In order for these changes to occur the metal must be heated above its recrystallization temperature, which depends on the composition of the metal. The annealing process is sometimes called a recrystallization anneal, though other names like process anneal are also used.
During a recrystallization anneal new grains form in the metal. The final grain size depends on the annealing temperature and annealing time. For a particular annealing temperature, as the time at the temperature increases the grain size increases. For a particular annealing time, as the temperature increases the grain size increases. A particular alloy with larger grains has lower strength and more ductility than the same alloy with smaller grains.
The figure shows micrographs of a brass alloy that was 50% cold-rolled and annealed at two different temperatures. The cold-rolled sample had a yield strength of 550 MPa (80 ksi). The sample that was annealed at 550 °C (1022 °F) for 1 hour had a yield strength of 75 MPa (11 ksi). The sample that was annealed at 650 °C (1202 °F) for 1 hour and had a yield strength of 60 MPa (9 ksi).
Recrystallization annealing is also used as a final processing step to produce metal sheet, plate, wire, or bar with specific mechanical properties. Control of the annealing temperature and time, heating rate up to the annealing temperature, and amount of cold-working prior to anneal is important for obtaining the desired grain size, and therefore the desired mechanical properties. Control of the grain size is also important for metal that will be undergo significant forming, such as deep drawing, to prevent orange peel, a cosmetic defect.
Arrhenius' equation gives the dependence of the rate constant
 of a chemical reaction on the absolute
temperature
of a chemical reaction on the absolute
temperature  (in kelvins), where
(in kelvins), where is the pre-exponential factor (or simply the
prefactor),
is the pre-exponential factor (or simply the
prefactor),  is the activation energy, and
is the activation energy, and 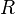 is the universal gas
constant:[1][2][3]
is the universal gas
constant:[1][2][3]

Alternatively, the equation may be expressed as

The only difference is the energy units of  : the former form uses energy per mole, which
is common in chemistry, while the latter form uses energy per
molecule directly, which is common in physics. The different units
are accounted for in using either the gas constant
: the former form uses energy per mole, which
is common in chemistry, while the latter form uses energy per
molecule directly, which is common in physics. The different units
are accounted for in using either the gas constant 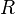 or the Boltzmann constant
or the Boltzmann constant 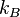 as the multiplier of temperature
as the multiplier of temperature  .
.
The units of the pre-exponential factor  are identical to those of the rate constant
and will vary depending on the order of the reaction. If the
reaction is first order it has the units s?1, and for
that reason it is often called the frequency factor or
attempt frequency of the reaction. Most simply,
are identical to those of the rate constant
and will vary depending on the order of the reaction. If the
reaction is first order it has the units s?1, and for
that reason it is often called the frequency factor or
attempt frequency of the reaction. Most simply,  is the number of collisions that result in a
reaction per second,
is the number of collisions that result in a
reaction per second,  is the number of collisions (leading to a
reaction or not) per second occurring with the proper orientation
to react[6] and
is the number of collisions (leading to a
reaction or not) per second occurring with the proper orientation
to react[6] and  is the probability that any given collision
will result in a reaction. It can be seen that either increasing
the temperature or decreasing the activation energy (for example
through the use of catalysts) will result in an increase in rate of
reaction.
is the probability that any given collision
will result in a reaction. It can be seen that either increasing
the temperature or decreasing the activation energy (for example
through the use of catalysts) will result in an increase in rate of
reaction.
Given the small temperature range kinetic studies occur in, it
is reasonable to approximate the activation energy as being
independent of the temperature. Similarly, under a wide range of
practical conditions, the weak temperature dependence of the
pre-exponential factor is negligible compared to the temperature
dependence of the  factor; except in the case of "barrierless"
diffusion-limited reactions, in which case the pre-exponential
factor is dominant and is directly observable.
factor; except in the case of "barrierless"
diffusion-limited reactions, in which case the pre-exponential
factor is dominant and is directly observable.
 According
to the Arrhenius equation, the kinetic constant increases as
temperature increases. At first, the value increases exponentially,
then it levels off as it approaches a limit. The units shown in
this graph are arbitrary.
According
to the Arrhenius equation, the kinetic constant increases as
temperature increases. At first, the value increases exponentially,
then it levels off as it approaches a limit. The units shown in
this graph are arbitrary.
The modified Arrhenius' equation[7] makes explicit the temperature dependence of the pre-exponential factor. The modified equation is usually of the form

The original Arrhenius expression above corresponds to n = 0. Fitted rate constants typically lie in the range -1<n<1. Theoretical analyses yield various predictions for n. It has been pointed out that "it is not feasible to establish, on the basis of temperature studies of the rate constant, whether the predicted T½ dependence of the pre-exponential factor is observed experimentally."[4] However, if additional evidence is available, from theory and/or from experiment (such as density dependence), there is no obstacle to incisive tests of the Arrhenius law.
Another common modification is the stretched exponential form:[citation needed]

where ? is a dimensionless number of order 1. This is typically regarded as a purely empirical correction or fudge factor to make the model fit the data, but can have theoretical meaning, for example showing the presence of a range of activation energies or in special cases like the Mott variable range hopping.
Poly(N-isopropylacrylamide) (PNIPA) microgels may offer several advantages over PNIPA-modified surfaces when used as sorbents in temperature-sensitive chromatography. Yet, a full exploitation of these advantages requires a better understanding of the mechanisms controlling the separation process. As a model system, we have studied the binding of three proteins (bovine serum albumin (BSA), ovalbumin, and lysozyme) to PNIPA microgels. Binding experiments were conducted both below (25 °C) and above (37 °C) the volume phase transition temperature of the gel, Tc. The analysis of the binding isotherms has shown that although an average gel particle contained a larger amount of protein below the phase transition temperature, the concentration of the protein within the particle was higher above this temperature. These findings were attributed to changes in the binding loci due to temperature swings around Tc: whereas a sorption mechanism is dominant below this temperature, surface-adsorption was more important above it. A comparison between the three studied proteins has shown that below Tc the binding increases with a decrease in the molecular weight. On the other hand, no significant difference in the bound protein amounts was observed above the phase transition temperature. Our results imply that, despite the increase in the gelapos;s hydrophobicity above the phase transition temperature, the resolution in bioseparations based on PNIPA gels is not necessarily better above Tc.
Related Solutions
Which metal hardening mechanism is reversible by annealing (heat treatment) of the material? You are given...
When aging an aluminum alloy, the final hardness depends on time at the heat treatment temperature....
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago