Question
In: Chemistry
what is the lewis structure of (CH3 )2NH2CL? explain
what is the lewis structure of (CH3 )2NH2CL? explain
Solutions
Expert Solution
Answer – Given the molecule (CH3)2NH2Cl and need to draw the Lewis structure-
Total valance electrons = (4*1) + (1*8) + (5*1) + 7
= 24 electrons
Lewis structure –
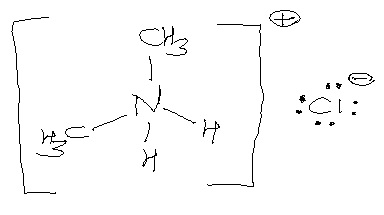
In this Lewis structure there are four bonding pair on the center atom N with +1 formal charge and there are 4 lone pair on the Cl with -1 charge, so overall molecule is neutral.
Formal charge on the N = 5 – ½ *8-0
= +1
Formal charge on the Cl = 7 – ½ *0-8
= -1
The molecular geometry and electronic geometry is tetrahedral.
Related Solutions
CH3+ lewis structure
Part ADraw the Lewis Structure for CH3+Part BWhat is the formal charge for each atom in the structure?
Lewis Structure of (CH3)3N -complete
Lewis Structure of (CH3)3N -complete
Draw Lewis dot structure (CH3)4 NCL A SALT
Draw Lewis dot structure (CH3)4 NCL A SALT
Which of these has hydrogen bonding capabilities (HINT: Draw Lewis Structure) CH3OH CH3-O-CH3 F2 CH3CH3 (CH3)3N...
Which of these has hydrogen bonding capabilities (HINT: Draw
Lewis Structure)
CH3OH CH3-O-CH3 F2 CH3CH3 (CH3)3N H2O
CIRCLE ALL THAT APPLY
Draw the molecular formula, partially condensed structure, Lewis structure, and bond line structure of CHCOCH2CH(CH3)2
Draw the molecular formula, partially condensed structure, Lewis
structure, and bond line structure of
CHCOCH2CH(CH3)2
In the compound (CH3)3N=CH2, how is the lewis structure formed, considering that the octet rule must...
In the compound (CH3)3N=CH2, how is the lewis structure formed,
considering that the octet rule must be maintained, but only one of
the three CH3's actually become a CH2? Also, is the reason why
(CH3)3N(+) - (-C:)H2 not permissible because the Carbon atom on CH2
has too many electrons, and the cation should be on Nitrogen
instead? This is in regards to Organic Chemistry (10th Ed) Problem
19P in chapter 1.
What is the Lewis structure of C2H6?
What is the Lewis structure of C2H6?
For each of the following compounds draw an acceptable Lewis dot structure (CH3CH2)2CHCO2CH(CH3)2 HSO4 - sulfur...
For each of the following compounds draw an acceptable Lewis dot
structure
(CH3CH2)2CHCO2CH(CH3)2
HSO4 -
sulfur tetrafluoride
bromine trichloride
dinitrogen pentoxide
(CH3)2SO
CH2Cl2
What is the correct Lewis structure for PF3?
What is the correct Lewis structure for
PF3? Non-bonding electrons not shown on outer
atoms
Classify each of the following as a Lewis acid or a Lewis base. CO2 P(CH3)3 H2O
Classify each of the following as a Lewis acid or a Lewis base.
Drag the appropriate items to their respective bins.
1) CO2
2)P(CH3)3
3)H2O
4)B(CH3)3
5)FE3+
6)CN-
7)OH-
8)H+
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
ADVERTISEMENT
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago