Questions
Please use financial statement information for Fin 602 Corp below for question 1) to 6). Income...
Please use financial statement information for Fin 602 Corp below for question 1) to 6).
Income statement (million dollars)
|
2019 |
2018 |
|
|
Sales |
2400 |
1000 |
|
Operating costs excluding depreciation |
2000 |
850 |
|
Depreciation |
270 |
25 |
|
Earnings before interest and taxes |
130 |
125 |
|
Less interest |
110 |
20.2 |
|
Earnings before tax |
20 |
104.8 |
|
Tax (21%) |
4.2 |
22 |
|
Net Income available to common stockholders |
15.8 |
82.8 |
|
Common dividend |
4.0 |
4.4 |
Balance sheet (million dollars)
|
2019 |
2018 |
|
|
Cash and equivalents |
12 |
10 |
|
Short term investments |
23 |
20 |
|
Account receivable |
350 |
150 |
|
Inventories |
300 |
200 |
|
Total current assets |
685 |
380 |
|
Net plant and equipment |
2173.5 |
250 |
|
Total assets |
2858.5 |
630 |
|
Accounts payable |
108 |
90 |
|
Notes payable |
90 |
71.5 |
|
Accruals |
72 |
60 |
|
Total current liabilities |
270 |
221.5 |
|
Long term debts |
2330 |
150 |
|
Total liabilities |
2600 |
371.5 |
|
Common stock (50 million shares) |
50 |
50 |
|
Retained earnings |
208.5 |
208.5 |
|
Common equity |
258.5 |
258.5 |
|
Total liabilities and equity |
2858.5 |
630 |
- Calculate NOPAT (net operating profit after tax) of the year 2019 and 2018
- Calculate the amount of total (net) operating capital for both year – 2019 and 2018
- Calculate the free cash flow for the year of 2019
- Calculate ROIC (Return on capital invested) and CR (Capital requirement ratio) for 2019 and 2018. Then interpret those ratios
- Basing on answers in 1) to 4), does Fin 602 look good in 2019? If not good, what looks like happening in operation and finance?
- Any suggestion to improve free cash flow or performance of Fin 602 Corp
In: Accounting
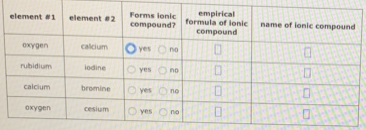
Decide whether each pair of elements in the table below will form an ionic compound.
Decide whether each pair of elements in the table below will form an ionic compound. If they will write the empirical formula and name of the compound formed in the spaces provided.
In: Chemistry
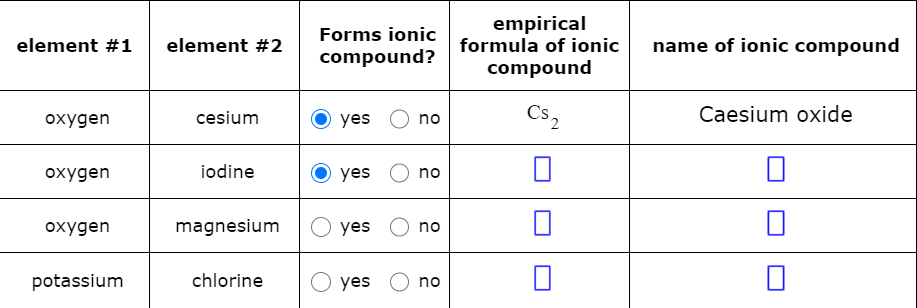
Decide whether each pair of elements in the table below will form an ionic compound.
Decide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided.
In: Chemistry
a 15.04 gram sample of chromium is heated in the presence of excess iodine. a metal...
a 15.04 gram sample of chromium is heated in the
presence of excess iodine. a metal iodide is formed with a mass of
125.2g. determine the empirical formula of the metal
iodide
In: Chemistry
A compound contains 5.80 mass % H, 20.16 mass % N, 23.02 mass % O, and...
| A compound contains 5.80 mass % H, 20.16 mass % N, 23.02 mass % O, and 51.02 mass % Cl. What is its empirical formula? |
In: Chemistry
Political Economy Question: Describe the empirical trends leading up to the 2008 crisis-was it caused by...
Political Economy Question:
Describe the empirical trends leading up to the 2008 crisis-was it caused by the financial crisis or was the financial crisis a crisis within a crisis?
In: Economics
Upon combustion, a compound containing only carbon and hydrogen produced 0.660 g CO2 and 0.270 g...
Upon combustion, a compound containing only carbon and hydrogen produced 0.660 g CO2 and 0.270 g H2O.
Find the empirical formula of the compound.
In: Chemistry
Flip a coin 30 times and document the result of each toss. Then provide both the...
Flip a coin 30 times and document the result of each toss. Then provide both the empirical and theoretical probability distributions. Use the appropriate expression for probability.
In: Statistics and Probability
flip a coin 30 times and document the result of each toss. Then provide both empirical...
In: Statistics and Probability
a. What is data mining? b. What is specification searching? c. Engaging in such behavior when...
a. What is data mining?
b. What is specification searching?
c. Engaging in such behavior when conducting empirical research is generally viewed negatively? Why?
In: Statistics and Probability